Beer-Lambert Law
Definition: Beer-Lambert Law is the linear relationship between absorbance of electromagnetic radiation and the concentration of an absorber in the medium through which the radiation travels. The general Beer-Lambert Law would be: 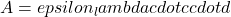 where A is the measured absorbance (i.e.
where A is the measured absorbance (i.e. 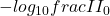 , I is the intensity of the measured light and
, I is the intensity of the measured light and 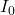 is the intensity of the incident light),
is the intensity of the incident light), 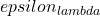 is the wavelength-dependent molar (decadic) absorption coefficient (formerly molar decadic extinction coefficient) with units of
is the wavelength-dependent molar (decadic) absorption coefficient (formerly molar decadic extinction coefficient) with units of 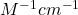 , c is the concentration of the absorber (
, c is the concentration of the absorber (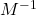 ) and d is the pathlength of light (cm). Please note that the subscript
) and d is the pathlength of light (cm). Please note that the subscript 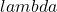 is often dropped with the understanding that the value for
is often dropped with the understanding that the value for 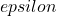 is for a specific wavelength.
Alternative definition:
Synonym: Beer-Lambert-Bouguer Law
References: https://doi.org/10.1002/cphc.202000464
https://doi.org/10.1017/CBO9781139029797
Related terms: modified Beer Lambert law, optical density, molar absorption coefficient
is for a specific wavelength.
Alternative definition:
Synonym: Beer-Lambert-Bouguer Law
References: https://doi.org/10.1002/cphc.202000464
https://doi.org/10.1017/CBO9781139029797
Related terms: modified Beer Lambert law, optical density, molar absorption coefficient
