Concentration
Definition: Concentration is the amount of a pure substance (solute) of interest within the amount of the whole substance (solution), and can be quantified as: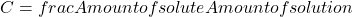 Concentration can be expressed in several ways. For example, molarity is a way of expressing concentration relating the number of particles of solute (expressed in moles) and the volume of the solution (expressed in liters). The unit of molarity is
Concentration can be expressed in several ways. For example, molarity is a way of expressing concentration relating the number of particles of solute (expressed in moles) and the volume of the solution (expressed in liters). The unit of molarity is 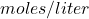 , sometimes denoted by molar or M. Within fNIRS it is common to see changes in concentration expressed in square brackets, such as [
, sometimes denoted by molar or M. Within fNIRS it is common to see changes in concentration expressed in square brackets, such as [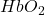 ].
Others include mass, number or volume concentration referring to the relation of the mass, the number of entities or the volume of the solute and the volume of the solution.
Alternative definition:
Synonym:
References: https://goldbook.iupac.org/terms/view/A00295
https://doi.org/10.1351/goldbook.A00295
Related terms:
].
Others include mass, number or volume concentration referring to the relation of the mass, the number of entities or the volume of the solute and the volume of the solution.
Alternative definition:
Synonym:
References: https://goldbook.iupac.org/terms/view/A00295
https://doi.org/10.1351/goldbook.A00295
Related terms:
